Report of local side effects following meningococcal B vaccine (Bexsero)
I believe that the new meningococcal B vaccine, named Bexsero, is another important brick in the routine vaccination of children living in endemic areas, mainly in babies under one-year-old.
My specific references and the reasons for the recommendation of the vaccine are discussed in detail on this website in another chapter, read more here.
But you have to read whats next before…
What are the known side effects of Bexsero?
The vaccine has local side effects which are less acknowledged to my opinion, and it is worthwhile to reference them precisely and to be aware of them before (and after), vaccinating.
In advance, it is known that this vaccine has relatively higher incidence of fever and local side effects (mainly local sensitivity at the injection site), higher incidence than that of other routine vaccines.
For this reason, this is the first time where it was recommended in some countries to administer Paracetamol to vaccine recipients under the age of one year.
Up to this point, all is fine and clear.
However, in my opinion, and based on my clinical observation (and I am clearly aware of the limitations posed by one physician’s observation), the incidence of these side effects is higher and stronger than expected.
I shall emphasize and emphasize again later – effects following vaccination or local sensitivity are not, in my opinion, reasons not to vaccinate. But parents as well as physicians must know and be familiar with them in order to give better counselling, before and after vaccination.
What is my experience with Bexsero (meningococcal B vaccine) vaccine?
In the beginning of the introduction of the vaccine in my country, on the 17 September 2019, I reported to the Ministry of Health of 4 cases of intense local reaction, including sensitivity and local swelling, which lasted even up to 4 weeks in vaccine recipients. I attached pictures to the intended form “Report of side effects following vaccination”.
I also reported about these effects to the local representative of the company that manufactures vaccines, GSK. The company, on its behalf, also reported to the Israeli Ministry of Health.
In my view, the incidence is higher than expected and reported by the vaccine manufacturer.
Since this report, I have seen many other similar cases.
Regarding fever – most cases were accompanied by fever and restlessness of up to 24 hours after vaccination – I suppose that this is a reaction to one of the vaccine ingredients. Not concerning at all.
What is that local reaction to Bexsero? Why is it happening?
Regarding the local reaction, it is worthwhile to note several important details, which I have made clearer with my observations:
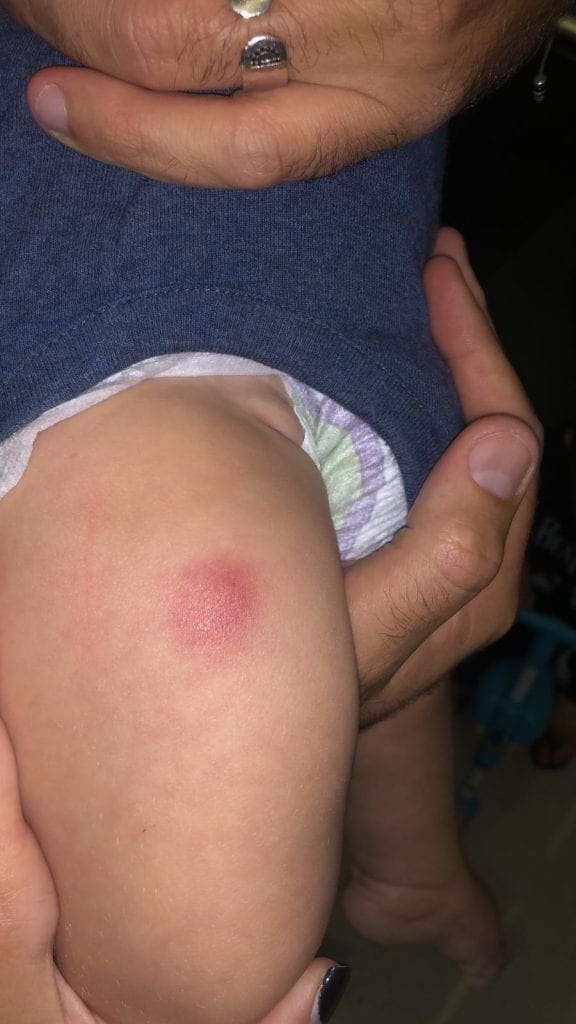
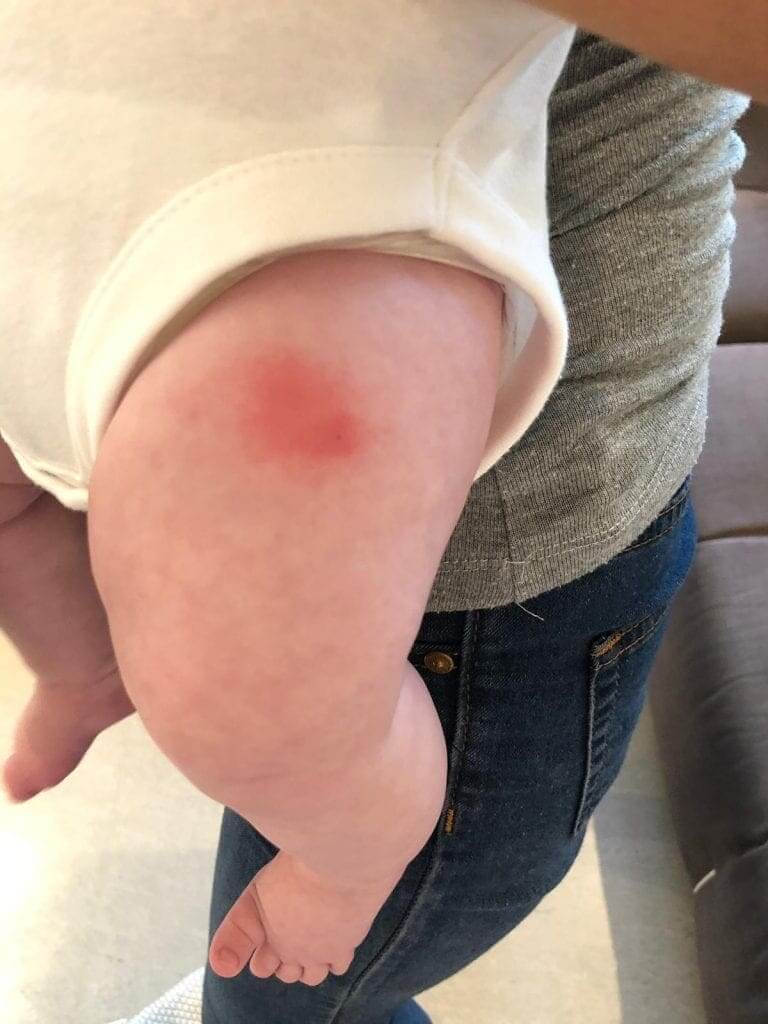
1. This is a relatively large and unpleasant local reaction (not usually seen with other routine vaccines), characterized by the appearance of local redness and extreme sensitivity in a non-small circumference around the injection site (please see two pictures attached). The reaction subsides without any special treatment within a number of days/a week, although a lump may remain even 4 weeks after administration.
2. In one child, who then received a repeated dose from me after the first dose – similar reaction was obtained, stronger than the first one.
So what could be the reasons for Bexsero local reaction?
Infection – although very rare after vaccination, in light of the fact that the vaccination site appears warm, red and swollen, some get confused by this appearance and administer antibiotics. The four cases I reported to the Ministry of Health, and the dozens more since then, were not treated with antibiotics and this cannot be an infection.
Bleeding – sometimes, the needle in intramuscular vaccine passes through a venous blood vessel and blood leaks to the muscle region. The body’s local reaction to the bleeding region also gives local signs of inflammation. However, it is uncommon and not possible that these four (and other non-reported), cases with this exact vaccine were caused from bleeding. This is too random.
Immunological local reaction to one of the preparation ingredients – local reaction to the vaccine or one of its ingredients is known after vaccination; From a mild local reaction that might follow many vaccines, up to a more significant reaction called Arthus Reaction. Some vaccines are more renowned as causes of such reaction (Tetanus, for instance), and, even though there are not many reports of such reactions in scientific literature, anyone who deals with vaccines, administers vaccines and closely monitors their patients, has seen this reaction, to a certain extent, several times before.
I must say that these four cases remind me of this reaction.
In the case where I vaccinated another child with the same vaccine (second dose), the local reaction came faster and was stronger. This item is also reminiscent of this type of immunological reaction. Diagnosis can be done by skin (and skin blood vessels) biopsy, but there was obviously no reason to do so in these cases.
I searched the scientific literature and have not found many reports of this reaction after Bexsero vaccination.
I received an informal reply form the Ministry of Health, stating that they are waiting to see whether there are abnormal reports from other places following this vaccination. Unfortunately, it is clear to me that the chance that anyone else reports systematically is low.
So, what are the final conclusions and what do I want to say?
It is appropriate that parents and caretakers (mainly pediatricians and family health center nurses) should be made aware of this local reaction.
– Rare cases of significant local reaction should be examined by the attending doctor. In most cases, warm compresses and patience resolve the matter, although the reaction may last several weeks.
– In my opinion it is right to continue vaccinating with this important vaccine. In those cases where children had a powerful (even if local) reaction with this vaccine, it is appropriate to give great consideration as to whether to administer the next dose.
– Anyone who has come across this local reaction, caretaker or parent, in real time or retroactively, should report to the regional health bureau by completing the local designated form.
– Only regulated reporting of anyone encountering this side effect will result in a correct estimate and accurate epidemiological investigation of this side effect.
Thank you for reading and sharing.
For comments and questions, please register
