
Bexsero – the new type-B meningococcal bacteria vaccine
Up until a few years ago, there were no vaccines against meningococcus type B (there were vaccines for other serotypes but not B).
In 2013, a novel vaccine against type B meningococcus named Bexsero, was manufactured by GSK and subsequently approved by the EMA and the FDA.
Another vaccine manufactured by Pfizer, named Trumenba was approved by the FDA in 2014, however it is indicated for individuals older than 10 years only and not for babies and small children.
In this chapter, I will try to answer all the questions regarding this vaccine and specify the population of babies in which this vaccine would be appropriate.
What is meningococcal disease?
The illness id caused by a bacteria called Neisseria meningitidis. This bacteria is part of the normal flora (whicj is the bacteria that normally resides in the nose and the pharynx), in up to 10% of adults and 25% of children, which are considered carries.
Rarely, this bacteria translocate from the oral cavity and may invade our body causing an invasive disease, including bloodstream infections (sepsis), central nervous system infections (meningitis) and more.
In light of the fact that the preliminary signs of a rapidly severe and deadly infection in children are non-specific, this bacteria was named by the popular media as “the violent bacteria”.
Who are the risk groups for invasive meningococcal disease?
In general, everyone.
However, some groups are at a greater risk for morbidity and mortality. These groups include:
# Children younger than 5 years, and mainly those which are younger than 2 years.
# Children or adults with specific, defined immune system disorders.
How does the meningococcus bacteria spread from one person to another?
The bacteria spread from one person to another through exposure to oral cavity or respiratory secretions of a carrier or a patient.
It should be emphasized that the high rate of carriers in population indicates that there is a substantial spread of bacteria from one person to another, and that most of the exposed people will become carriers and will not develop disease.
Only a minority of people carrying the bacteria in their nasopharynx will develop invasive disease.
What is the deal with the various strains (types) of this bacteria? What is type-B?
There are several strains/types of meningococcus differentiating one from another by the presence of different sugars on their capsule surrounding the bacteria.
The strains that cause most of the illness in humans are A/C/Y/W135 and strain B. Strain B is the main cause of disease in various countries, especially in small children.
There are other conjugate vaccines (“new generation” vaccines that are more efficient than the old polysaccharide vaccines), directed against the other 4 strains of meningococcus (A/C/Y/W135). These vaccines are recommended as routine vaccines in selected countries and are also administered to various defined populations (mainly people with immune system disorders or travelers).
This chapter does not discuss these vaccines at all, but only discusses the vaccine against meningococcus B.
What is the incidence of meningococcal B disease in children?
The incidence of meningococcus B is mostly related to your geographic region of living. There are countries with many cases per years and countries with almost no morbidity from the bacteria.
It is known that children younger than 5 years and mainly babies under the age of two year constitute the vast majority of cases.
While most cases are sporadic, this bacterium can cause also outbreaks, especially in institutes with close contact such as child care centers, schools and more.
Is there any experience with this vaccine around the world?
Definitely. In September 2015 the vaccine was introduced into the routine vaccination program in England, due to significant morbidity from meningococcal disease.
In England, the vaccine is routinely administered to babies younger than age 1 year of according to this schedule: first dose at the age of two months, second at the age of 4 months and third at 1 year. All doses administered alongside the routine vaccines.
Other countries that vaccinate routinely are Ireland, Italy, Lithuania, New-Zealand, Spain, Andorra and more.
Other countries have recommended receiving the vaccine, including Australia, Austria, Belgium, Canada, Israel, France, Germany, Portugal and more.
We should note that morbidity from strain B differs from one continent to another, and from one country to another. Therefore, recommendation can be found based on epidemiological data regarding local morbidity and age distribution in a specific country.
Is the Bexsero vaccine efficient?
There are several ways to assess the efficacy of a vaccine. I have chosen to give the example, the best one in my opinion, of England, which was the first country to introduce the vaccine into the routine vaccination program.
After 10 months of vaccination, a reduction of approximately 50% was demonstrated in the number of cases.
In a work published in January 2020, in the best clinical journal in the world (NEJM), it was found that vaccine efficacy in disease prevention was 52.7% following 2 vaccine doses, and 59.1% after a third dose at the age of 1 year. For further reading, the article is summarized in the following link.
It is reasonable to expect that in the longer-run the reduction in disease rates will be higher.
So, is it justified to vaccinate with a vaccine that reduces morbidity by 50%?
This is, of course, the great question, and I assume that many parents who have reached the point of reading this chapter, were looking for the answer to this question.
The answer depends of course on geography. If you are living in a region with high incidence of Meningococcus B disease, then your ministry of health might have already decided for you and placed the vaccine in the routine baby care visits.
Remember, that even if the disease is not common, and it is true that the vaccine, as we will later demonstrate, has it’s limitations (logistics, cost, side effects), this vaccine can prevent an invasive deadly disease. In my opinion it would be appropriate to overcome the surrounding limitations.
So, who should get the Bexsero vaccine?
Most of the countries that recommended this vaccine aimed it to be given to children in the first years of life, mostly under 1 year.
However, vaccination can obviously be done at a later age.
Vaccination is recommended for any age for certain populations suffering from specific immune system disorders (HIV carriers, spleen function problems or people treated with a drug called Eculizumab and more).
Who can receive the Bexsero vaccine?
Any baby starting from the age of 2 months.
Who cannot be vaccinated?
People/children with known sensitivity to one of the vaccine components.
Where is the Bexsero vaccine administered?
The vaccine is administered intramuscularly, into the thigh or arm region, depending on the patient’s age.
How many doses are required in order to be vaccinated?
Unfortunately, this is a bit complicated, since it depends upon the risk factors (whether this is a healthy baby/child or one with underlying disease) and upon the age of initiation of vaccination.
In babies with a healthy background who initiate vaccination as per the standard, up to the age of 6 months, there is room for 3 doses – two doses two months apart and a third booster dose after at least 6 months, and preferably at the ages 12-15 months (in any case the booster dose is recommended for administration before the age of 2 years).
When the course of vaccines is initiated at a different age (older than 6 months) or in the event of a child with underlying diseases, the numer of doses and their time gaps varies.
Please see, for your convenience, a table defining the number of doses and the minimal time gaps in between them in healthy children without risk factors.
*
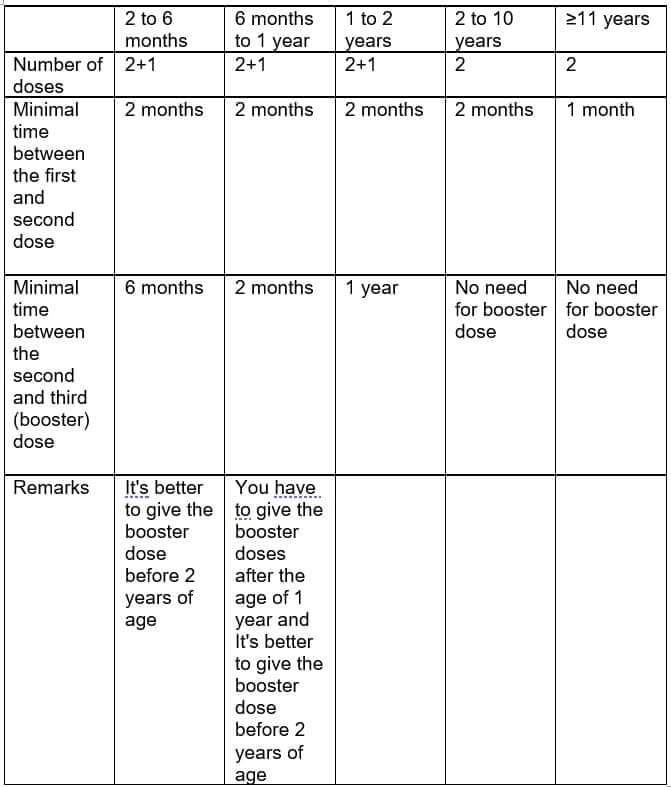
What is the expected duration of protection for babies following this course of vaccination?
The assessment is that administration of 2 doses provides protection for babies for approximately 1.5 years and receiving a booster dose provides protection for over 3 years. In adolescents, the duration of protection is even longer (over 7 years).
Since, as mentioned earlier, most of the morbidity is caused in babies, toddlers and small children, the vaccine is expected to protect them precisely during this important time period.
What are the Bexsero side effects?
As with any vaccine, local side effects such as redness and injection site sensitivity are the most common.
Fever and restlessness have been more commonly observed when the vaccine was administered at the same time with the other routine vaccines.
In light of the relatively high incidence of these side effects, even though not dangerous, in some countries it is recommended to administer 3 doses of Paracetamol to vaccine recipients under the age of 1 year. first dose within 1 hour from vaccine administration and then two more doses 4-6 hours apart.
Over two year ago, after launching Bexsero vaccine in my country (Israel), I reported to the Israeli Ministry of Health on the higher-than-expected rate and severity of local side effects of this vaccine; for further reading please follow the following link. This potential local side effects are important to recognize, but, at the end of the day, this is not a reason not to vaccinate. Since that report I saw dozens of other alike cases. They all recovered without any need for specific treatment.
How and when can the administration of this vaccine be combined with other routine vaccines?
In many countries it is permissible to administer this vaccine along with all other routine vaccines, at the same time or with any time gap before or afterwards. The vaccines must be administered in different anatomical regions. However, and as mentioned earlier, the incidence of mild side effects were observed at higher incidence when the vaccine was administered along with the other routine vaccines. Therefore, other countries recommended a separation of at least 3 days between the other routine vaccines and this vaccine, in vaccine recipients under the age of 1 year.
How can the Bexsero vaccine be administered, in practice?
The answer depends on your local practice.
If it is a mandatory vaccine in your country / region, then your child will receive the vaccine in the routine baby care visits.
If it is only recommended in your country and was not implemented part of the routine vaccination program in your baby care visits, ask the nurse or your pediatrician.
So what do we conclude?
After the successful vaccination of millions children in England, and after the distribution of more than 50 million doses around the world, my impression is that vaccine is another important stage in protecting our children’s health. I hope that this chapter will help parents reach the right decision concerning this vaccine in their young child.
For comments and questions, please register
